Worth to know
Learn more
 It shows that stainless steel is one of most durable materials used in many industries. What to do however, when processing this material at client facility, it does not fulfill high expectations of final customer. These may be low surface resistance to specific chemicals, hard to remove surface contaminations, or when special certificates regarding surface properties are required. Most of these deficiencies may be overcome by ECM Sp. z o.o. with its staff of specialists experienced in solving such problems. According to the identified specific problem, it can advise final finishing process, optimized with regard to cost economy and quality, and satisfying even the most demanding customer.
It shows that stainless steel is one of most durable materials used in many industries. What to do however, when processing this material at client facility, it does not fulfill high expectations of final customer. These may be low surface resistance to specific chemicals, hard to remove surface contaminations, or when special certificates regarding surface properties are required. Most of these deficiencies may be overcome by ECM Sp. z o.o. with its staff of specialists experienced in solving such problems. According to the identified specific problem, it can advise final finishing process, optimized with regard to cost economy and quality, and satisfying even the most demanding customer.Processes
Pickling of steel with higher chromium and nickel content is used to obtain clean article surface, well protected against corrosion. In course of process all: surface material layer, after welding tint, residues of mechanical grinding, welding scale, oils and greases are removed. As result of chemical reactions, hydrogen is liberated, which facilitates pealing and chipping-off oxides, produced during welding. Time of pickling depends mainly from steel chemical composition and process temperature. Pickling bath temperature can speed up process (higher temperature) or retard it (lower temperature). Visually, pickled article gets a mat-grey surface. Pickling may be a final stage of processing of article surface, or just an initial stage prior to electrochemical polishing process. Articles may be pickled if the steel has 18% of chromium and 8% of nickel, as a minimum.
In full scale system , pickling is carried using mixture of two acids ? hydrofluoric acid and nitric acid with strong oxidizing properties. Additionally to optimize processing, organic inhibitors are added. The process itself can be carried in 3 possible ways:
- Immersion pickling – article is placed into processing tank filled with acid mixture.
- Spray pickling – pickling mixture is sprayed on whole surface of article using low pressure equipment ? in this case there is no limit to article size because of tank size.
- Gel method – weld seams are brushed with use of thick pickling paste (gel).
When pickling is finished, processed articles must be thoroughly rinsed. After pickling, rinsing water ought to be neutralized with adequate basic solution and resulting sludge transferred to specialized firm for utilization.
II. Passivation of stainless and acid resisting steels
During passivation process metal surface is made passive and its standard potential increases. This effect is produced by building on metal surface an oxide layer. In case of noble steel this layer consist mainly of chromium oxides. Passivation of stainless and acid resisting steels may take place in two ways. It may be spontaneous ? occurring in environment with oxygen content (i.e. in air or well oxygenized water) or forced ? occurring under action of strongly oxidizing acid, like nitrogen acid. While created spontaneously passive layer is relatively thin, the layer produced by nitric acid is several times thicker and more uniform. Thanks to that, metal surface gets excellent coating protecting it against corrosion and other chemical influences that may degrade its surface. Passivated surface is self repairing i.e. when slightly damaged, for example if scratched, then after some time damaged place shall be covered by new oxide layer. In case of pickling process, structure of surface changes, however chemical passivation does not produce macroscopic changes, the only effect can be increased resistance.
Nearly all grades of stainless steel can be passivated. The only condition is chromium content, not less than 10-11%. In case of fragile articles of high accuracy, to passivating bath inhibitors are added, to prevent any interference to article structure, from nitric acid.
Passivation can be carried similarly like pickling, i.e.
- by immersion method
- by spraying method
- by gel method.
To assure correct and efficient passivation process, article surface should be properly prepared i.e. degreased, residues of eventual grinding removed and welding seams cleaned mechanically or by pickling. The better preparation of surface, the better final result of passivation. Articles after passivation, possess finally formed anticorrosion layer, already just after rinsing in water.
The best passive layer with excellent anticorrosion properties and considerable hardness is produced in electropolishing process.
III. Electrochemical polishing of steel
Electrochemical polishing of steel can be carried out in various baths, however large scale process requires electrolyte mixture consisting of sulfuric and phosphoric acids. Historically this process started to be used barely after the Second Word War, for various grades of carbon and alloy steels. Best technical and economical results have been obtained for acid resisting steels, which are very tiresome in mechanical polishing.
Typical arrangement for electrochemical polishing of steel is shown in fig.1
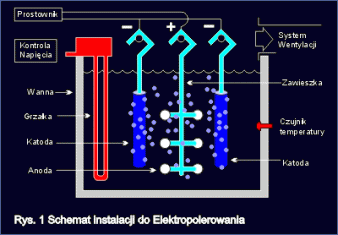
Electrochemical polishing process is carried in electrolytic tanks or other tanks filled with proper bath ? acid mixture. Polished article (anode) is immersed in the bath and connected to positive end of direct current supply. Inside the tank, there are cathodes, connected to negative end of current supply. Cathodes and anodes immersed into proper bath make closed electrical circuit. When current flows through this circuit, preserving adequate process parameters, there follows first smoothing and finally polishing of the surface of electropolished article. Quantity of metal removed in the process, is in proportion to current density flowing.
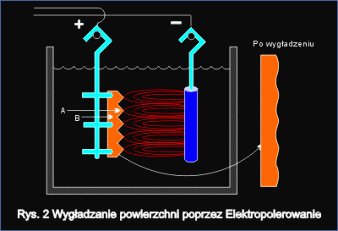
Smoothing of surface
Fig.2 shows surface smoothing mechanism during electrochemical polishing. Initially metal surface is coarse. There are micro protrusions (A) and micro recesses (B) with sharp edges. During the electropolishing process micro protrusions peaks are intensively dissolved, while in micro pits area, this dissolving is much slower. As a result of such dissolving mechanism, processed surface gets smoother with sharp edges rounded. As a result of electrochemical polishing the material gets the highest resistance to corrosion.